The Red Trouser Day charity are pleased to announce approval of funding for Dr Irene Chong’s project into the identification of rectal cancer radiotherapy response.
This study aims to improve the experience of patients, families and friends, and may reduce their stress and anxiety if identified molecular markers from this project could help to direct treatment and indicate whether it is safe for them to defer surgery and potentially avoid a permanent stoma.
In addition, if a radioresistant molecular profile is identified, it is possible that future patients could have alternative treatment that spares them the unnecessary toxicity of radiation.
Aims / Objectives:
To use molecular profiling of rectal cancers to identify candidate biomarkers that could ultimately be used to direct the effective use of nCRT.
Introduction and background:
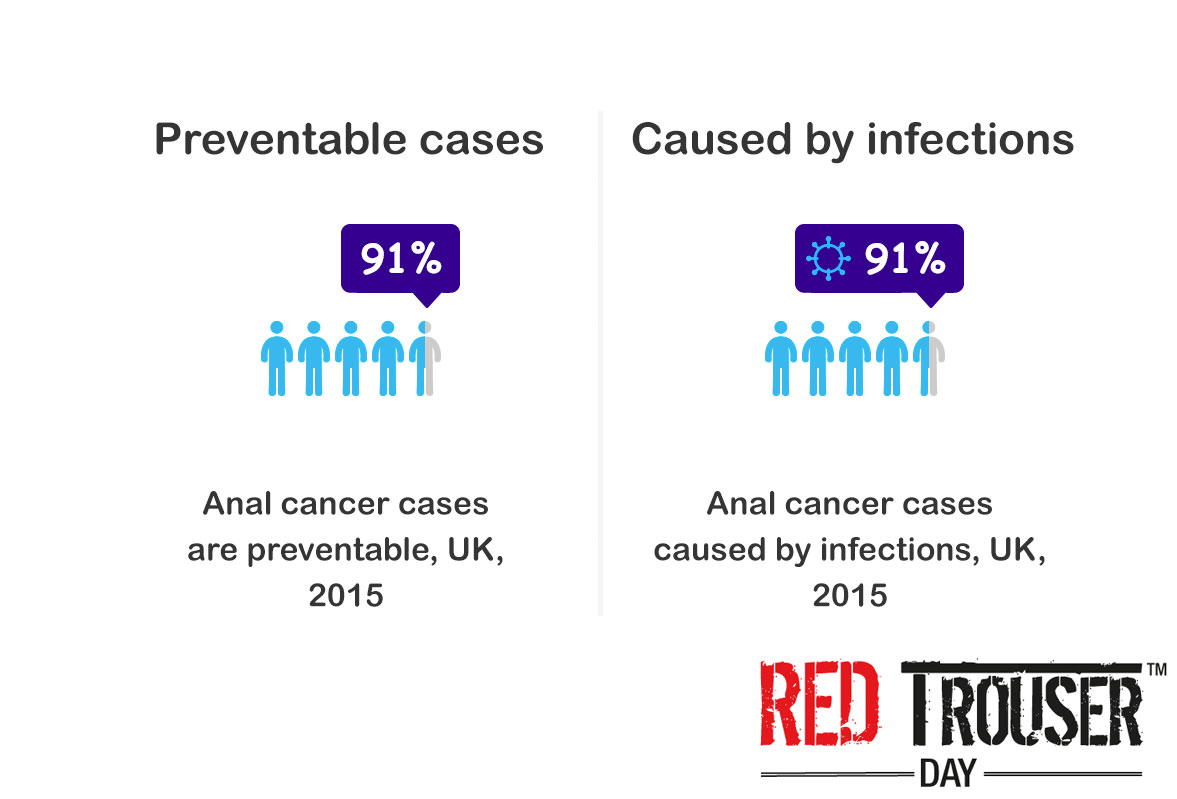
Neoadjuvant ChemoRadioTherapy (nCRT) improves local control and often results in tumour downstaging for patients with locally advanced rectal cancer. nCRT may lead to significant rates of pathological complete response (pCR) in the region of 10-15% and selected patients with clinical and radiological evidence of complete response to nCRT can be managed non-operatively, including the possibility of avoiding a permanent stoma. However, approximately 20% of tumours are intrinsically radioresistant and these patients suffer the side effects of radiotherapy unnecessarily; currently there are no reliable biomarkers to distinguish patients that are likely to achieve a good response from those that will not.
A number of small, heterogeneous studies that aim to identify molecular biomarkers with the potential to predict response to nCRT have been previously reported, although none have been reliably validated. While activation of the host immune response plays an important role in the therapeutic effects of chemoradiation, it can also activate immune suppressive pathways.
At the Royal Marsden Hospital and the Pelican Cancer Foundation, we have now completed a clinical study, the Deferral of Surgery Trial, which has investigated the safety of a non-operative approach for complete clinical and radiological responders. Rectal cancer patients on this trial first receive chemoradiotherapy, after which they are stratified according to MRI and an MDT discussion; those with stable/progressive disease are referred for surgery, whilst those with no MRI visible tumour or visible tumour but a partial response to CRT are have their disease monitored by MRI or FDG/PET, with the option of later CRT and/or surgery depending upon subsequent progression of disease.
Over 100 patients, many of whom were referred from external cancer centres, have now been managed using this approach. As part of this trial, patients are monitored with serial clinical, endoscopic and radiological examinations for a total follow up of 10 years for each patient, providing a rich source of material and data that could be used to address my central aim. 20-30% of patients exhibit tumour regrowth despite an initial good response to CRT, suggesting radioresistant tumour clones emerge in these cases, who then undergo subsequent surgery.
In addition, I have direct access to tissue within the 6 vs 12 study (NCT01037049) assessing whether greater downstaging and tumour regression is observed when surgery is delayed to 12 weeks after completion of nCRT compared to 6 weeks. These are unique prospective dataset with mature follow up and represents an opportunity to identify molecular markers of radiosensitivity as well as resistant clones in tumour that develop in patients who experience tumour re-growth.
Outline of Plan:
” The work I propose will build on existing prospective rectal cancer clinical trials where I am translational lead that have complete sets of clinical and radiological outcomes, as well as baseline tumour tissue, resection specimens and blood collection.
Focusing on molecular processes that have previously been shown to modulate response to radiotherapy, I will utilise tissue from the afore mentioned trials to assess genetic determinants of DNA damage repair, hypoxia, immune regulation and proliferation. Given that radiological and histopathological response is available for all patients recruited to clinical trials, I will compare the molecular profiles of radiosensitive tumours and compare these with those that are re-grow or are radioresistant.
I will collaborate with Professor Alan Melchor within the Centre for Translational Immunotherapy to assess the role of the immune microenvironment in radioresistance within these trial tissues using a differential expression analysis of immune-related genes and tumour associated stroma in radiotherapy resistance. Using a RNA sequencing platform, each tumour will be classified into a colorectal molecular subtype (CMS) and I will assess whether CMS1, enriched for inflammatory and immune cells, predicts for response to nCRT for patients with rectal cancer. “
About Dr Irene Chong
Dr Irene Chong is a Clinical Oncology Consultant at The Royal Marsden NHS Foundation Trust and a Clinician Scientist at The Institute of Cancer Research. She trained at St Bartholomew’s Medical College, London, and undertook her PhD in Cancer Biology at the ICR. She specialises in the treatment of upper and lower gastrointestinal malignancies.
Dr Chong works in The Royal Marsden’s Gastrointestinal Clinical Trials Unit where she develops biomarker-driven, proof-of-concept clinical trial protocols based on discoveries made in the laboratory. Her laboratory research aims to discover new therapeutic targets and biomarkers for oesophageal and rectal cancers through DNA sequencing and functional profiling.
Dr Chong received several awards during her PhD, including the University of London Scholarship Award, the Fourth NIHR Infrastructure Experimental Medicine Research Award, and the Royal College of Radiologists Kay Visiting Award.